4.5.18. Improving the H\(_2^+\) Variational Approximation#
4.5.18.1. Motivation:#
The linear combination of atomic orbital (LCAO) description of the H\(_2^+\) molecule that we saw in previous notes gives a qualitatively correct but quantitatively inaccurate picture of bond formation. In these notes, we will discuss ways of improving the basis functions to yield more quantitively correct results.
4.5.18.2. Learning Goals:#
After working through these notes, you will be able to:
Define the difference between a Hydrogen-atom orbital and a Slater orbital
Describe how using Slater 1s orbitals allows us to get an improved variational result for the H\(_2^+\) molecule as compared to hydrogen-atom 1s orbitals
Describe what it means to include polarization functions in atomic orbitals.
4.5.18.3. Coding Concepts:#
The following coding concepts are used in this notebook:
4.5.18.4. Background#
We saw in the previous notes that the variational solution to H\(_2^+\) in a minimal basis (hydrogen atom 1s basis functions on each nucleus) yields a qualitatively correct picture of covalent bond formation but is not a good quantitative match with the excact solution. Specifically, the predicted bond length is 2.5 Bohr in the minimal basis approximation but is 2.0 in the exact solution. Additionally, the bond energy is -0.064 Hartree in the minimal basis and just over -0.1 Hartree in the exact solution.
There are two obvious ways to improve the variational solution:
Increase the basis set size
Improve the basis functions
In these notes we will address option two. We will do so in two ways: first by adding a variational parameter to the basis functions themselves and second by including polarization functions.
4.5.18.5. Including Variational Parameters in the Basis Functions#
In the previous notes we used a trial wave function of
where \(1s_A\) denotes a hydrogen atom 1s wave function centered on nucleus \(A\) and is given by the function
In our variational example of the helium atom we introduced the idea of using the effective nuclear charge as a variational parameter. We will do so again here. This is a fairly general procedure and the hydrogen atom functions with nuclear charge as a variable (e.g. as \(Z\) or \(\zeta\)) are called Slater orbitals. The Slater 1s orbital is
We will thus propose the following wave function
If you recall from our discussion of the variational solution in the minimal basis, there was no need to determine \(c_1\) and \(c_2\) using the variational approach since it can be argued that their magnitudes should be the same and these magnitudes can be determined by normalization. These same arguments hold here (despite introducing the \(Z\) variable). Thus, the normalized trial wave function is
where \(S(Z,R)\) is the overlap integral between the two basis functions. That is
You will show for homework that this integral is
Using this trial wave function it can be shown that
Differentiating \(E_\psi\) w.r.t \(Z\), setting to zero, and solving for \(Z_{min}\) yields
Note that this is only strictly true at the value for \(R\) for which this is determined (\(R=2.0\) Bohr). Technically \(E_{min}\) should be minimized w.r.t. \(Z\) at every value of \(R\).
Below I plot \(E\) as a function of \(R\) and determine the minimum \(Z\) at each \(R\) value using a numeric minimzation routine. I also plot the minimal basis result.
Show code cell source
from scipy.optimize import minimize
import matplotlib.pyplot as plt
%matplotlib inline
import numpy as np
def J(R):
return np.exp(-2*R)*(1+1/R) - 1/R
def K(R):
return -np.exp(-R)*(1+R)
def S(R):
return np.exp(-R)*(1+ R + R*R/3)
def E(Z,R):
T = Z**2/2-Z**2*(S(Z*R)/2 + K(Z*R))
T /= (1+S(Z*R))
V = -Z + Z*J(Z*R) + 2*Z*K(Z*R)
V /= (1+S(Z*R))
V += 1.0/R
return T + V
R = np.arange(1,8,0.001)
plt.figure(figsize=(8,6),dpi= 80, facecolor='w', edgecolor='k')
plt.tick_params(axis='both',labelsize=20)
plt.grid( which='major', axis='both', color='#808080', linestyle='--')
E_1s_basis = np.empty(R.size)
E_slater_basis = np.empty(R.size)
Z_min = np.empty(R.size)
for i, r in enumerate(R):
E_1s_basis[i] = E(1,r)
Z_min[i] = minimize(E,1.0,args=(r)).x[0]
E_slater_basis[i] = E(Z_min[i],r)
plt.plot(R,E_1s_basis,lw=4, label=r'E - 1s basis')
plt.plot(R,E_slater_basis,lw=4, label=r'E - Slater basis')
plt.xlabel(r'R/$a_0$',fontsize=20)
plt.ylabel(r'$E_0 (Hartree)$',fontsize=20)
#plt.ylim(-0.6,-0.2)
plt.legend(fontsize=20)
plt.title(r'Variational Solution to the H$_2^+$ Molecule in Slater Basis',fontsize=16)
plt.tight_layout()
plt.show();
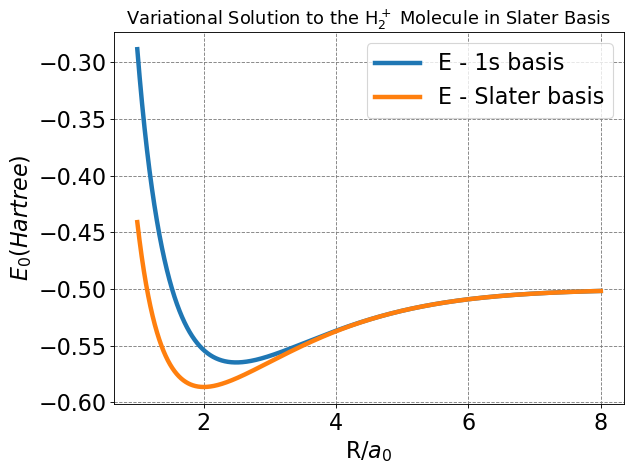
We see that allowing the function to be modified at each value of \(R\) to take on a specific effective nuclear charge allows the variational solution to lower and to become much closer to the exact value. The value of the bond distance, in particular, is dramatically improved over the 1s minimal basis solution.
R = np.arange(1,8,0.001)
plt.figure(figsize=(8,6),dpi= 80, facecolor='w', edgecolor='k')
plt.tick_params(axis='both',labelsize=20)
plt.grid( which='major', axis='both', color='#808080', linestyle='--')
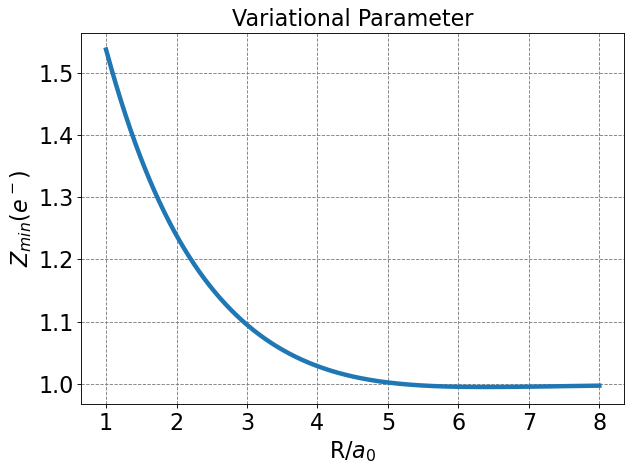
plt.plot(R, Z_min,lw=4, label=r'$Z_{min}$')
plt.xlabel(r'R/$a_0$',fontsize=20)
plt.ylabel(r'$Z_{min} (e^-)$',fontsize=20)
plt.title(r'Variational Parameter',fontsize=20)
plt.tight_layout()
plt.show();
# make two plots of the same spherical harmonic
from mpl_toolkits.mplot3d import Axes3D
from matplotlib import cm, colors
import numpy as np
import matplotlib.pyplot as plt
from scipy.special import sph_harm
from scipy.special import eval_genlaguerre
from scipy.special import lpmv
from scipy.special import factorial
%matplotlib inline
from scipy.optimize import root
a0 = 1.0 # radial unit of Bohr!
def hydrogen_atom_wf(r,theta,phi,n,l,m):
Y_norm = np.sqrt((2*l+1)*factorial(l-np.abs(m))/(4*np.pi*factorial(l+np.abs(m))))
R_prefactor = -np.sqrt(factorial(n-l-1)/(2*n*factorial(n+l)))*(2.0/(n*a0))**(l+1.5)*np.power(r,l)*np.exp(-r/(n*a0))
R = R_prefactor*eval_genlaguerre(n-l-1,2*l+1,2*r/(n*a0))
return Y_norm*sph_harm(m, l, phi, theta).real*R
def h_1s(r):
R_prefactor = 1/np.pi
R = R_prefactor*np.exp(-r)
return R
def slater_1s(r,Z):
R_prefactor = Z**3/np.pi
R = R_prefactor*np.exp(-Z*r)
return R
def h2plus_prob(r_nuc,x,y,z):
r1 = np.sqrt(x*x + y*y + z*z)
phi1 = np.arctan2(y,x)
theta1 = np.arccos(z/r1)
r2 = np.sqrt((x-r_nuc)**2 + y*y + z*z)
phi2 = np.arctan2(y,x-r_nuc)
theta2 = np.arccos(z/r2)
#
s12 = np.exp(-r_nuc)*(1+r_nuc+r_nuc**2/3)
#
h2plus_wf = np.sqrt(1/(2*(1+s12)))*(h_1s(r1) + h_1s(r2))
return h2plus_wf**2
def h2plus_slater_prob(r_nuc, Z, x,y,z):
r1 = np.sqrt(x*x + y*y + z*z)
phi1 = np.arctan2(y,x)
theta1 = np.arccos(z/r1)
r2 = np.sqrt((x-r_nuc)**2 + y*y + z*z)
phi2 = np.arctan2(y,x-r_nuc)
theta2 = np.arccos(z/r2)
#
s12 = np.exp(-Z*r_nuc)*(1+Z*r_nuc+Z**2*r_nuc**2/3)
#
h2plus_wf = np.sqrt(1/(2*(1+s12)))*(slater_1s(r1,Z) + slater_1s(r2,Z))
return h2plus_wf**2
def plot_h2plus_prob(r_nuc, ax_obj, r=np.linspace(0,2,20), theta=np.linspace(0,np.pi,20), phi=np.linspace(0,2*np.pi,25)):
R, THETA, PHI = np.meshgrid(r, theta, phi)
R = R.flatten()
THETA = THETA.flatten()
PHI = PHI.flatten()
x = R*np.sin(THETA)*np.cos(PHI)
y = R*np.sin(THETA)*np.sin(PHI)
z = R*np.cos(THETA)
x = np.concatenate((x,x+r_nuc))
y = np.concatenate((y,y))
z = np.concatenate((z,z))
wf = h2plus_prob(r_nuc,x,y,z)
vmax = max(np.abs(np.amin(wf)),np.abs(np.amax(wf)))
vmin = -vmax
# plot
ax_obj.set_title(rf'$R={r_nuc}$', fontsize=18)
ax_obj.scatter3D(x,y,z,c=wf, cmap='RdBu', vmin=vmin, vmax=vmax,alpha=0.25)
#ax_obj.set_box_aspect((100,100,100))
ax_obj.set_axis_off()
ax_obj.axes.xaxis.set_ticklabels([])
ax_obj.axes.yaxis.set_ticklabels([])
ax_obj.axes.zaxis.set_ticklabels([])
def plot_h2plus_prob_xz_projection(r_nuc, ax_obj):
x = np.linspace(-2.5,8,1200)
z = np.linspace(-2,2,600)
X, Z= np.meshgrid(x, z)
Y = np.zeros(X.shape)
x = X.flatten()
y = Y.flatten()
z = Z.flatten()
wf = h2plus_prob(r_nuc,x,y,z)
wf = wf.reshape(X.shape)
vmax = max(np.abs(np.amin(wf)),np.abs(np.amax(wf)))
vmin = -vmax
# plot
ax_obj.set_title(rf'$R={r_nuc}$ 1s', fontsize=18)
#c = ax_obj.pcolormesh(X, Z, wf, cmap='RdBu', vmin=vmin, vmax=vmax)
c = ax_obj.pcolormesh(X, Z, wf, cmap='hot_r',vmin=0,vmax=vmax)
# set the limits of the plot to the limits of the data
ax_obj.axis([-2.5, 8, -2, 2])
ax_obj.set_aspect('equal')
#ax_obj.set_axis_off()
return c
def plot_h2plus_slater_prob_xz_projection(r_nuc, Zmin, ax_obj):
x = np.linspace(-2.5,8,1200)
z = np.linspace(-2,2,600)
X, Z= np.meshgrid(x, z)
Y = np.zeros(X.shape)
x = X.flatten()
y = Y.flatten()
z = Z.flatten()
wf = h2plus_slater_prob(r_nuc,Zmin,x,y,z)
wf = wf.reshape(X.shape)
vmax = max(np.abs(np.amin(wf)),np.abs(np.amax(wf)))
vmin = -vmax
# plot
ax_obj.set_title(rf'$R={r_nuc}$ Slater', fontsize=18)
#c = ax_obj.pcolormesh(X, Z, wf, cmap='RdBu', vmin=vmin, vmax=vmax)
c = ax_obj.pcolormesh(X, Z, wf, cmap='hot_r',vmin=0,vmax=vmax)
# set the limits of the plot to the limits of the data
ax_obj.axis([-2.5, 8, -2, 2])
ax_obj.set_aspect('equal')
#ax_obj.set_axis_off()
return c
fig, ax = plt.subplots(6,2,figsize=(8,12),dpi= 80, facecolor='w', edgecolor='k')
R = np.array([6.0,3.5,3.0,2.5,2.0,1.5])
for i, r in enumerate(R):
plot_h2plus_prob_xz_projection(r,ax[i,0])
Z_min = minimize(E,1.0,args=(r)).x[0]
plot_h2plus_slater_prob_xz_projection(r, Z_min, ax[i,1])
plt.tight_layout()
plt.show();
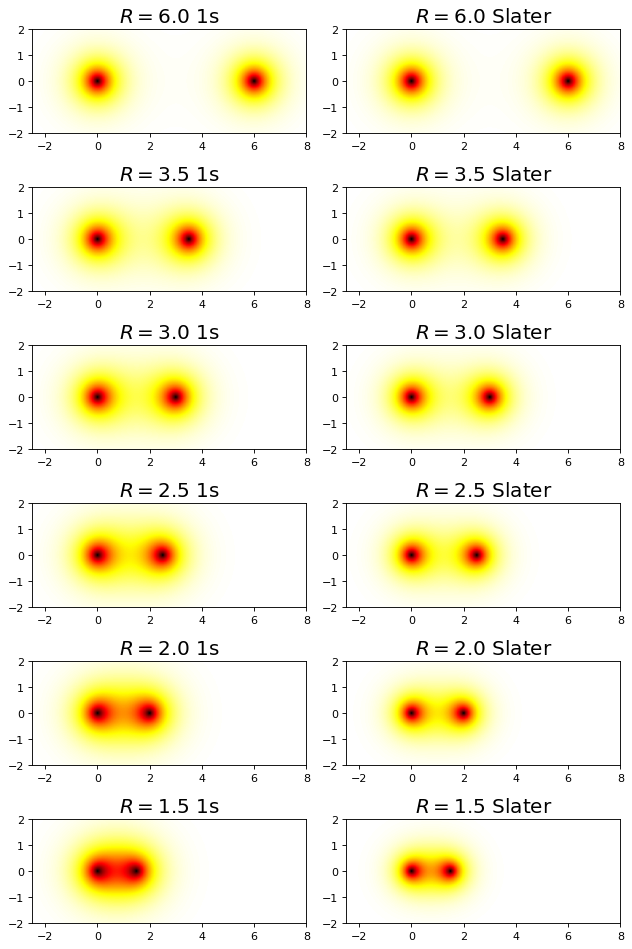
4.5.18.5.1. Including Polarization Functions#
In the previous example we modified the effective nuclear charge for a 1s-like orbital and saw significant improvement in doing so. The spherical symmetry of the 1s orbital is still maintained in this process. If we think about what our intuition tells us that a \(\sigma\) bonding orbital will look like in this case, we would not consider it having spherical symmetry about each nucleus. We might anticapte a large lobe between the two nuclei and small lobes on the opposing side. This intuition is likely built on my own experiences reading chemistry text books etc but perhaps some of it is more general?
Regardless, it is likely the spherically symmetric behavior of a 1s orbital is not ideal to create a molecular covalent bond. Thus, it is natural to hypothesize that basis functions that allow the breaking of the spherical symmetry and the creation of a lobe of density between the two nuclei to yield better results for the H\(_2^+\) variational solution.
We must remember, however, that the 1s orbital is the correct solution at infinte separation. Thus, a good basis will still allow for this behavior while becoming non-spherically symmetric as the two nuclei become close together. We will achieve this behavior by adding a \(p_z\) oribtal to the 1s orbital and allow for the amount of \(p_z\) character to be determined at every \(R\) value.
Our trial wave function will thus look like
where
Notice that we can treat the effective nuclear charge for the 1s and 2p\(_z\) parts as separate variational parameters. Additionally, we will treat \(a\), the amount of \(p_z\) character in the polarized orbital, to be a variational parameter as well. Below I plot the following polarized orbital for various values of \(a\):
Show code cell source
# make two plots of the same spherical harmonic
from mpl_toolkits.mplot3d import Axes3D
from matplotlib import cm, colors
import numpy as np
import matplotlib.pyplot as plt
from scipy.special import sph_harm
from scipy.special import eval_genlaguerre
from scipy.special import lpmv
from scipy.special import factorial
%matplotlib inline
from scipy.optimize import root
a0 = 1.0 # radial unit of Bohr!
def hydrogen_atom_wf(r,theta,phi,n,l,m):
Y_norm = np.sqrt((2*l+1)*factorial(l-np.abs(m))/(4*np.pi*factorial(l+np.abs(m))))
R_prefactor = -np.sqrt(factorial(n-l-1)/(2*n*factorial(n+l)))*(2.0/(n*a0))**(l+1.5)*np.power(r,l)*np.exp(-r/(n*a0))
R = R_prefactor*eval_genlaguerre(n-l-1,2*l+1,2*r/(n*a0))
return Y_norm*sph_harm(m, l, phi, theta).real*R
def plot_polarize_hydrogen_atom_wf_xz_projection(n1,l1,m1,n2,l2,m2,a,ax_obj):
x = np.linspace(-10,10,1000)
z = np.linspace(-10,10,1000)
X, Z= np.meshgrid(x, z)
Y = np.zeros(X.shape)
R = np.sqrt(X*X + Y*Y + Z*Z).flatten()
THETA = np.arccos(Z.flatten()/R)
PHI = np.arctan2(Y,X).flatten()
wf = np.zeros(R.shape)
wf = hydrogen_atom_wf(R,THETA,PHI,n1,l1,m1)
wf += a*hydrogen_atom_wf(R,THETA,PHI,n2,l2,m2)
wf = wf.reshape(X.shape)
vmax = max(np.abs(np.amin(wf)),np.abs(np.amax(wf)))
vmin = -vmax
# plot
ax_obj.set_title(rf'$a={a}$', fontsize=18)
c = ax_obj.pcolormesh(X, Z, wf, cmap='RdBu', vmin=vmin, vmax=vmax)
# set the limits of the plot to the limits of the data
ax_obj.axis([-10, 10, -10, 10])
ax_obj.set_aspect('equal')
#ax_obj.set_axis_off()
return c
fig, ax = plt.subplots(1,4,figsize=(12,4),dpi= 80, facecolor='w', edgecolor='k')
A = [0,1.0, 2.0, 10.0]
for i, a in enumerate(A):
plot_polarize_hydrogen_atom_wf_xz_projection(1,0,0,2,1,0,a, ax[i])
/var/folders/td/dll8n_kj4vd0zxjm0xd9m7740000gq/T/ipykernel_45652/1906438207.py:17: DeprecationWarning: `scipy.special.sph_harm` is deprecated as of SciPy 1.15.0 and will be removed in SciPy 1.17.0. Please use `scipy.special.sph_harm_y` instead.
return Y_norm*sph_harm(m, l, phi, theta).real*R
I will not solve this all the way out but it is doable. The integrals get even messier etc but are still all solvable. Instead I will just provide you with the results for the minimum energy and \(R\) value:
This value for the energy and bond distance are very close to the exact solution. The inclusion of the polarization dramatically improved the energy (though didn’t have such a dramatic impact on the bond distance) as compared to just varationally optimizing the 1s-like orbitals.


