4.3.8. Second Law of Thermodynamics#
import numpy as np
import matplotlib.pyplot as plt
%matplotlib inline
import plotting as myplt
---------------------------------------------------------------------------
ModuleNotFoundError Traceback (most recent call last)
Cell In[1], line 4
2 import matplotlib.pyplot as plt
3 get_ipython().run_line_magic('matplotlib', 'inline')
----> 4 import plotting as myplt
ModuleNotFoundError: No module named 'plotting'
Four equivalent statements of the 2nd Law:
Entropy is a state function that increases for all spontaneous processes (\(\Delta S_{univ} > 0\)). \(\Delta S = \int \frac{\delta q_{rev}}{T}\). The functin \(\frac{q}{T}\) is maximized for a reversible process.
Spontaneous changes are those from which work can be extracted. If done reversibly, the they yield a maximum amount of work.
Heat spontaneously flows from hot to cold and not in the reverse direction.
\(dS \geq \frac{\delta q}{T}\) where \(S\) is entropy and the equality holds for a reversible process.
4.3.8.1. Entropy is a State Function#
We will prove that an additional state function exists. We will call it entropy.
A state function other that internal energy and enthalpy must exist. The proof is as follows.
where the last equality is for a reversible process for which there is only \(PV\) work. Since the left-hand side of this equation is the differential of a state function (\(dU\)), we are free to follow any desired path. We now, however, dictate that the system is an ideal gas meaning \(dU = C_VdT\) and \(P=\frac{nRT}{B}\):
where I have solved the top equation for \(\delta q_{rev}\) to achieve the bottom equation. Now I will divide this bottom equation by \(T\) to achieve a separation of variables:
Integrating both sides of this equation yields
We note that the right hand side (rhs) of this equation is a state function. This can be inferred because it only depends on the final state (\(V_2\) and \(T_2\)) and initial state (\(V_1\) and \(T_1\)). Since the rhs of the equaution is a state function, the lhs of the equation must also be a state function. We call this the change in entropy:
or
4.3.8.2. Example#
Let’s consider an expansion/contraction of an ideal gas and compute the heat and entropy along two different paths
Compute \(q_{sys}\) and \(\Delta S_{sys}\) for the isothermal expansion of a gas from \(V_1\) to \(V_2=2V_1\) if done:
(a) Reversibly
(b) Against constant external pressure \(P_{ext} = P_2\)
(a) Reversible isothermal expansion from \(V_1\) to \(V_2=2V_1\):
Start by computing \(q_{sys}\):
Note that \(q_{sys}^{(a)}>0\) meaning that the surroundings are providing heat to the system to cause this expansion.
Now \(\Delta S\)
(b) Expansion from \(V_1\) to \(V_2\) against constant external pressure \(P_{ext} = P_2\)
Again, we start by computing \(q_{sys}^{(b)}\):
Now plug in \(P_2 = \frac{nRT}{V_2}\) and \(\Delta V = V_2 - V_1 = \frac{1}{2}V_2\):
Since entropy is a state function we still have:
Compare part (a) and part (b):
Entropies are identical since entropy is a state function.
Heat:
Which is bigger?
Thus we see that \(q\) is larger for the reversible process (a) than the irreversible process (b). Equivalently, \(-w\), the extractable work, is maximized in process (a).
4.3.8.3. Carnot Cycle (again)#
Compute the change in entropy (\(\Delta S\)) for each step along the Carnot cycle and show that \(\Delta S_{total} = 0\).
def plot_PV_diagram_adiabat(n=1,R=0.08206,V=np.arange(0.5,7.1,0.1),pUnit="atm",vUnit="L",T1=100,T2=200):
xlabel = "V (" + vUnit + ")"
ylabel = "P (" + pUnit + ")"
fig, ax = myplt.define_figure(xlabel=xlabel,ylabel=ylabel)
label = "T$_1$=" + str(T1)+" K"
ax.plot(V,n*R*T1/V,label=label,lw=4)
label = "T$_2$=" + str(T2)+" K"
ax.plot(V,n*R*T2/V,label=label,lw=4)
# adiabat 1
lowV = (T1*1.5**(2/3)/T2)**(3/2)
highV = 1.5
Vadiabat1 = np.arange(lowV,highV+0.1,0.1)
ax.plot(V,n*R*T1*1.5**(2/3)/V**(5/3),label="Adiabat 1",lw=4)
ax.plot(Vadiabat1,n*R*T1*1.5**(2/3)/Vadiabat1**(5/3),lw=1,c="k")
label = "(" + str(1.5) +","+ str(np.round(n*R*T1/1.5,decimals=1)) + "," +str(T1)+")"
label = "(V$_1$,P$_1$,T$_1$)"
ax.annotate(label,xy=(0.5,n*R*T1/1.5-1),fontsize=16)
plt.scatter(1.5,n*R*T1/1.5,s=50,color="tab:blue")
label = "(" + str(1.5) +","+ str(np.round(n*R*T2/1.5,decimals=1)) + "," +str(T2)+")"
label = "(V$_2$,P$_2$,T$_2$)"
plt.scatter(lowV,n*R*T2/lowV,s=50,color="tab:orange")
ax.annotate(label,xy=(lowV+0.05,n*R*T2/lowV),fontsize=16)
ax.annotate("A",xy=(0.4,n*R*T1/1.5+ 0.5*(n*R*T2/lowV-n*R*T1/1.5)),fontsize=24)
label = "(" + str(2.5) +","+ str(np.round(n*R*T2/2.5,decimals=1)) + "," +str(T2)+")"
label = "(V$_3$,P$_3$,T$_2$)"
# adiabat 2
lowV2 = 2.5
highV2 = (T2*2.5**(2/3)/T1)**(3/2)
Vadiabat2 = np.arange(lowV2,highV2+0.1,0.1)
ax.plot(V,n*R*T2*2.5**(2/3)/V**(5/3),label="Adiabat 2",lw=4)
ax.plot(Vadiabat2,n*R*T2*2.5**(2/3)/Vadiabat2**(5/3),lw=1,c="k")
plt.scatter(2.5,n*R*T2/2.5,s=50,color="tab:orange")
ax.annotate(label,xy=(2.6,n*R*T2/2.5),fontsize=16)
ax.annotate("B",xy=(1.5,n*R*T2/2+3),fontsize=24)
label = "(" + str(2.5) +","+ str(np.round(n*R*T1/2.5,decimals=1)) + "," +str(T1)+")"
label = "(V$_4$,P$_4$,T$_1$)"
plt.scatter(highV2,n*R*T1/highV2,s=50,color="tab:blue")
ax.annotate(label,xy=(highV2,n*R*T1/highV2),fontsize=16)
ax.annotate("C",xy=(4.25,3.0),fontsize=24)
ax.annotate("D",xy=(3,n*R*T1/2-3.0),fontsize=24)
#ax.annotate('',xytext=(1.5,n*R*T1/1.5),xy=(1.5,n*R*T2/1.5),arrowprops={'arrowstyle':"-",'lw': 2, 'color': 'black'})
#ax.annotate('',xytext=(2.5,n*R*T1/2.5),xy=(2.5,n*R*T2/2.5),arrowprops={'arrowstyle':"-",'lw': 2, 'color': 'black'})
#ax.annotate('',xy=(2.5,5.0),xytext=(2.5,5.1),arrowprops={'arrowstyle': 'simple','lw': 2, 'color': 'black'})
#ax.annotate('',xy=(1.5,8.2),xytext=(1.5,8.1),arrowprops={'arrowstyle': 'simple','lw': 2, 'color': 'black'})
vsub = np.arange(lowV,2.51,0.01)
ax.plot(vsub,n*R*T2/vsub,lw=1,c="k")
vsub = np.arange(1.5,highV2+0.01,0.01)
ax.plot(vsub,n*R*T1/vsub,lw=1,c="k")
ax.fill_between(np.arange(1.5,2.51,0.01),n*R*T1/np.arange(1.5,2.51,0.01),n*R*T2/np.arange(1.5,2.51,0.01),facecolor="purple",alpha=0.5,interpolate=True)
ax.fill_between(np.arange(lowV,1.51,0.01),n*R*T2/np.arange(lowV,1.51,0.01),n*R*T1*1.5**(2/3)/np.arange(lowV,1.51,0.01)**(5/3),facecolor="purple",alpha=0.5,interpolate=True)
ax.fill_between(np.arange(2.5,highV2+0.01,0.01),n*R*T2*2.5**(2/3)/np.arange(2.5,highV2+0.01,0.01)**(5/3),n*R*T1/np.arange(2.5,highV2+0.01,0.01),facecolor="purple",alpha=0.5,interpolate=True)
ax.set_ylim(0,35)
plt.legend(fontsize=16)
plot_PV_diagram_adiabat()
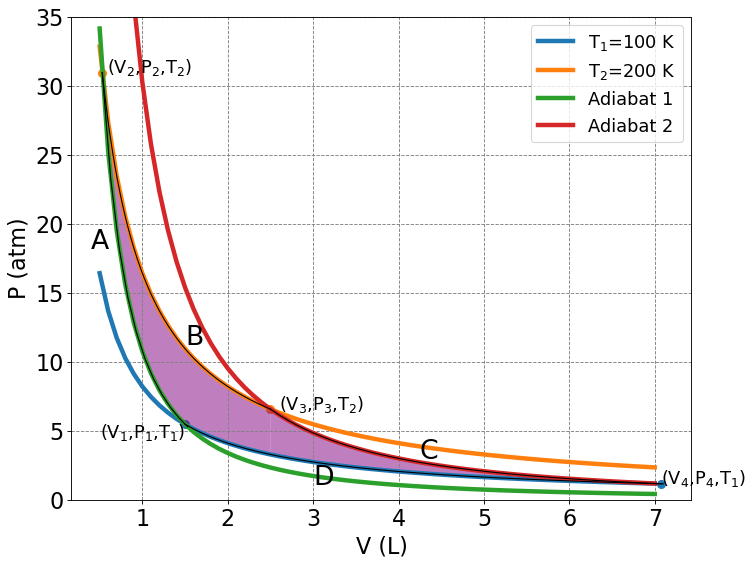
4.3.8.3.1. \(A\) : Reversible Adiabatic Contraction#
Since the process is reversible and \(q_A = 0\), we have
4.3.8.3.2. \(B\) : Reversible Isothermal Expansion#
Since the process is reversible and \(q_B = nRT_2\ln\left(\frac{V_3}{V_2}\right)\), we have
4.3.8.3.3. \(C\) : Reversible Adiabatic Expansion#
Since the process is reversible and \(q_C = 0\), we have
4.3.8.3.4. \(D\) : Reversible Isothermal Contraction#
Since the process is reversible and \(q_D = nRT_1\ln\left(\frac{V_1}{V_4}\right)\), we have
4.3.8.3.5. Summary#
Process |
\(w\) |
\(q\) |
\(\Delta U\) |
\(\Delta H\) |
\(\Delta S\) |
|---|---|---|---|---|---|
A - Adiabatic Contraction |
\(\frac{3}{2}nR(T_2-T_1)\) |
\(0\) |
\(\frac{3}{2}nR(T_2-T_1)\) |
\(\frac{5}{2}nR(T_2-T_1)\) |
\(0\) |
B - Isothermal Expansion |
\(nRT_2\ln\left(\frac{V_2}{V_3}\right)\) |
\(nRT_2\ln\left(\frac{V_3}{V_2}\right)\) |
\(0\) |
\(0\) |
\(nR\ln\left(\frac{V_3}{V_2}\right)\) |
C - Adiabatic Expansion |
\(\frac{3}{2}nR(T_1-T_2)\) |
\(0\) |
\(\frac{3}{2}nR(T_1-T_2)\) |
\(\frac{5}{2}nR(T_1-T_2)\) |
\(0\) |
D - Isothermal Contraction |
\(nRT_1\ln\left(\frac{V_4}{V_1}\right)\) |
\(nRT_1\ln\left(\frac{V_1}{V_4}\right)\) |
\(0\) |
\(0\) |
\(nR\ln\left(\frac{V_1}{V_4}\right)\) |
4.3.8.3.6. Efficiency#
For a heat engine (\(w_{total}<0\)), the efficiency is the amount of work extracted, \(w_{total}\), divided by the energy in put, \(q_{in}\).
\(\varepsilon = \frac{w_{total}}{q_{in}}\)
For the Carnot cycle, this becomes:
\(\varepsilon = \frac{T_h-T_c}{T_h}\)


